| 25G 26G 27G 28G 29G 30G |
Needle length | Thread length |

|
| 25mm | 30mm | ||
| 38mm | 60mm | ||
| 50mm | 80mm | ||
| 60mm | 100mm | ||
| 90mm | 160mm |
| 25G 26G 27G |
Needle length | Thread length |
|
| 38mm | 60mm | ||
| 50mm | 80mm | ||
| 60mm | 100mm | ||
| 70mm | 120mm | ||
| 90mm | 160mm |
| 23G 25G 26G |
Needle length | Thread length |

|
| 38mm | 60mm | ||
| 50mm | 80mm | ||
| 60mm | 100mm | ||
| 70mm | 120mm | ||
| 90mm | 160mm |
| 25G 26G 27G 28G 29G 30G |
Needle length | Thread length |
|
| 38mm | 60mm | ||
| 50mm | 80mm | ||
| 60mm | 100mm | ||
| 70mm | 120mm | ||
| 90mm | 160mm |
| 19G 21G 23G 25G |
Needle length | Thread length |
|
| 38mm | 60mm | ||
| 50mm | 80mm | ||
| 60mm | 100mm | ||
| 90mm | 150mm | ||
| 100mm | 150mm |

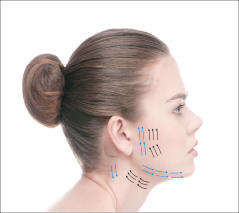

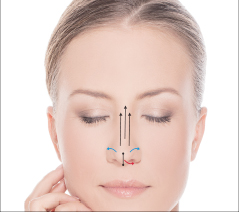
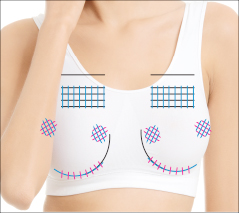

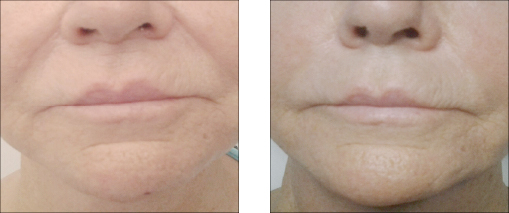
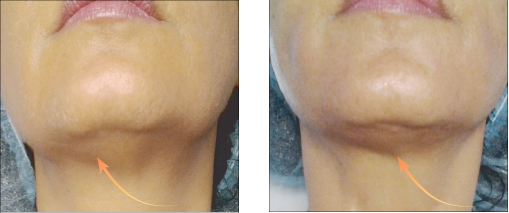
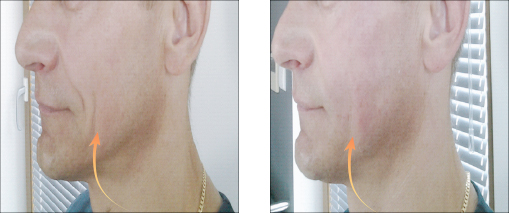
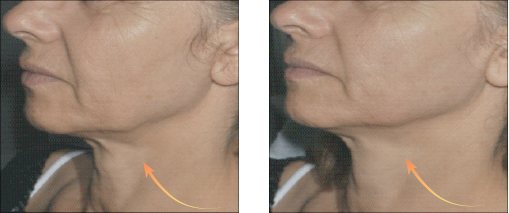
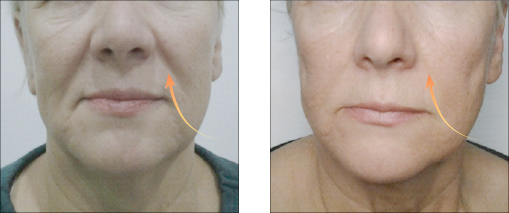
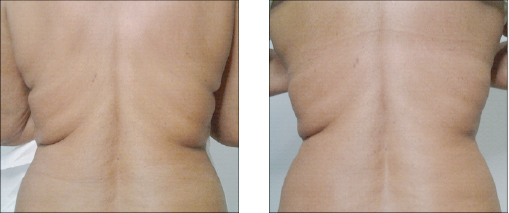 Vector Linear technique of the skin of lips before and after 14 days
Vector Linear technique of the skin of lips before and after 14 days| Model Name | Needle size | Needle Length | PDO length |
| Mono | 25g | 38 | 60 |
| 50 | 80 | ||
| 60 | 100 | ||
| 90 | 160 | ||
| 26G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 90 | 160 | ||
| 27G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 90 | 160 | ||
| 29G | 25 | 30 | |
| 38 | 60 | ||
| 50 | 80 | ||
| 60 | 100 | ||
| 30G | 25 | 30 | |
| 38 | 60 | ||
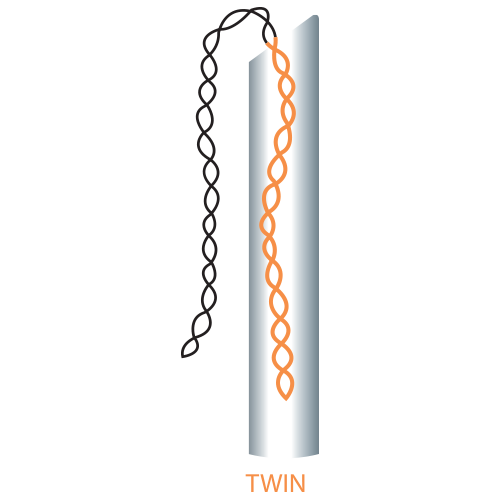
| Twin | 25G | 38 | 60 |
| 50 | 80 | ||
| 60 | 100 | ||
| 70 | 120 | ||
| 90 | 160 | ||
| 26G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 90 | 160 | ||
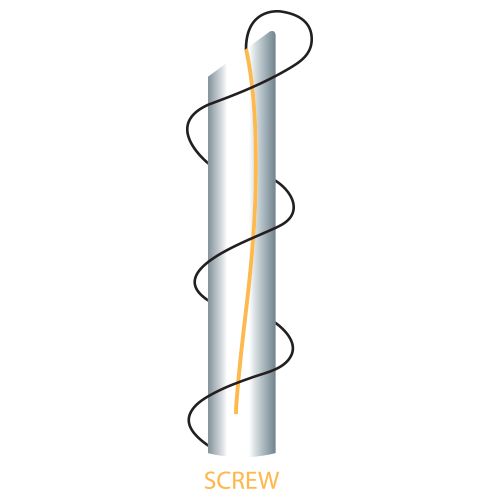
| Single Screw | 25G | 60 | 100 |
| 26G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 90 | 160 | ||
| 27G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 29G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 30G | 38 | 60 |
| Model Name | Needle size | Needle Length | PDO length |
| Double Screw | 23G | 70 | 120 |
| 25G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 26G | 38 | 60 | |
| 50 | 80 | ||
| 60 | 100 | ||
| 70 | 120 | ||
| 90 | 160 | ||
| 100 | 180 | ||
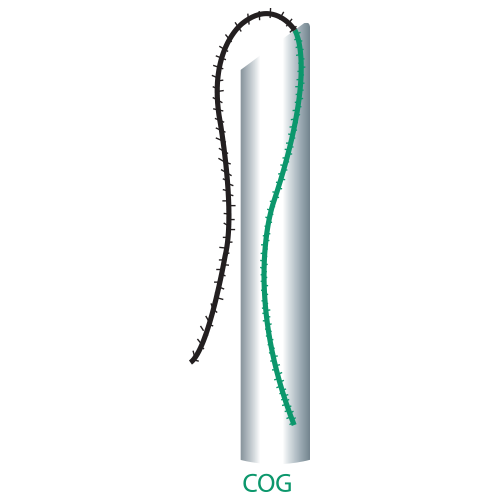
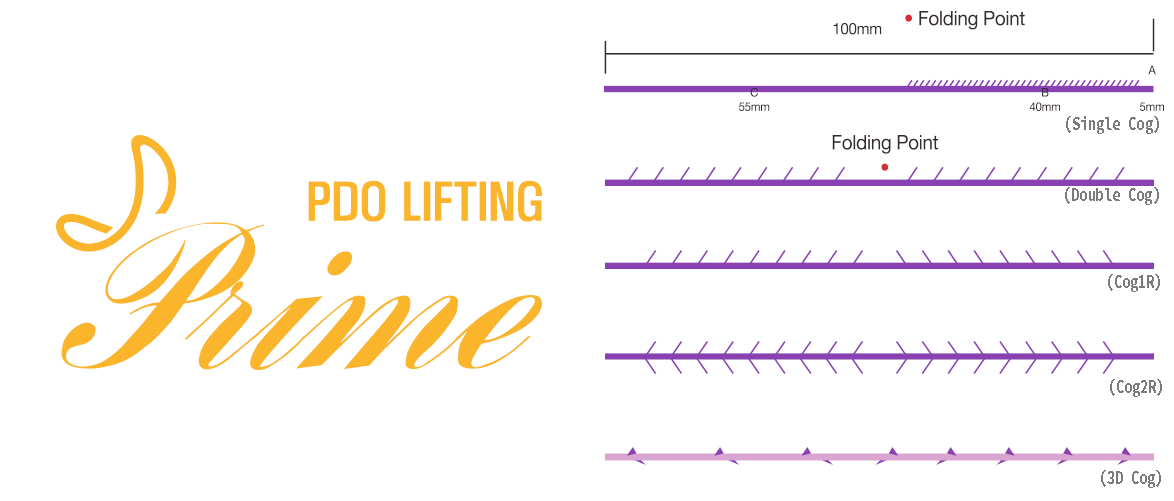
| Single Cog | 19G | 100 | 150 |
| 21G | 90 | 150 | |
| 23G | 38 | 50 | |
| 50 | 70 | ||
| 60 | 100 | ||
| 60 | 110 | ||
| 90 | 140 | ||
| 90 | 150 | ||
| 25G | 90 | 130 | |
| 90 | 150 | ||
| Double Cog | 19G | 100 | 150 |
| 21G | 90 | 150 | |
| 23G | 38 | 50 | |
| 50 | 70 | ||
| 60 | 100 | ||
| 60 | 110 | ||
| 90 | 130 | ||
| 90 | 140 | ||
| 90 | 150 | ||
| Cog 1R | 19G | 100 | 150 |
| 21G | 90 | 150 | |
| 23G | 90 | 150 | |
| Cog 2R | 19G | 100 | 150 |
| 21G | 90 | 150 | |
| 23G | 90 | 150 | |
| 3D Cog | 19G | 100 | 150 |
| 21G | 90 | 150 | |
| 23G | 90 | 150 |





CE Mark approval (Feb, 2012) · ClassⅢ
KFDA approval (May, 2013)
· Graft/prosthesis, biomaterial · B04230.01(4)
Usage of HA produced by bacterial fermentation (Streptococcus equi)
Biocompatibility · Non-toxic · Non-immunogenic
Biodegradability
BDDE cross-linked HA
· To overcome the short biodegradability of natural HA
· Improvement of the sustainability
HA Conc. : 20mg/ml (2%)
High viscosity gel : 2,700,000 cP
Injection Force : 10 N
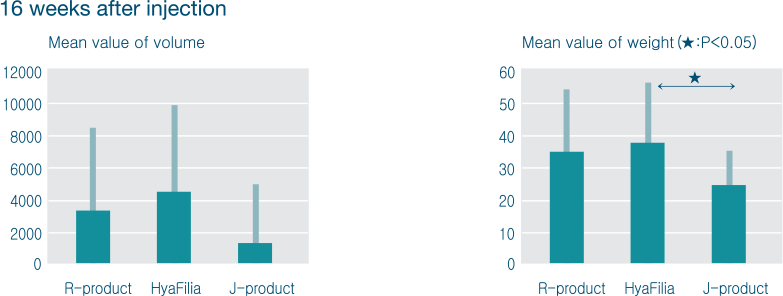
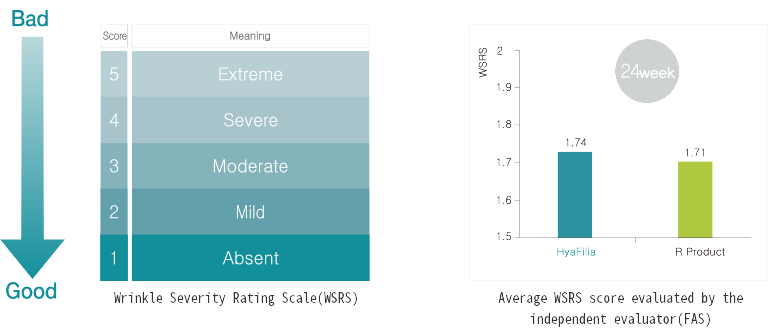
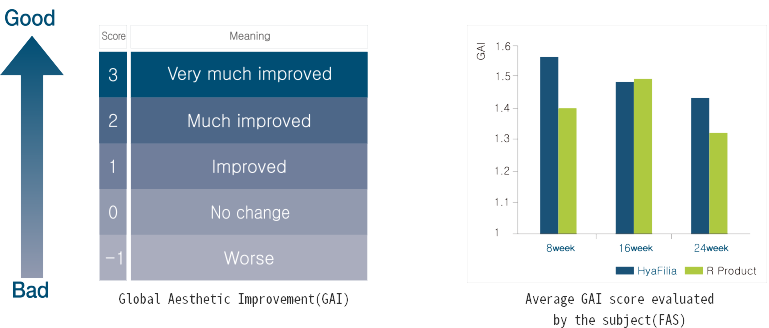
Through multicenter clinical trial, safety and efficacy of HyaFilia have been proven.
| Classification | HyaFilia | R-product | J-product |
|---|---|---|---|
| Composition | hyaluronic acid | hyaluronic acid | hyaluronic acid |
| Crosslinking agent | BDDE | BDDE | BDDE |
| Total HA concentration (g/ml) | 20 | 20 | 22~26 |
| Complex viscosity (cP) | 2.7 x 106 | 2.8 x 106 | 0.9 x 106 |
| Swelling ratio (ml/g) | 4 | 2.8 | 3.8 |
| Injection force (N) | 10 | 9 | 13 |
| Endotoxin (EU/ml) | Less than 0.25 | Less than 0.5 | - |
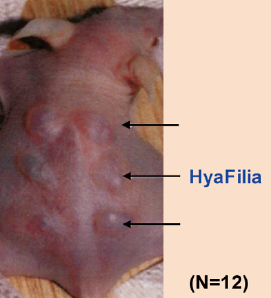
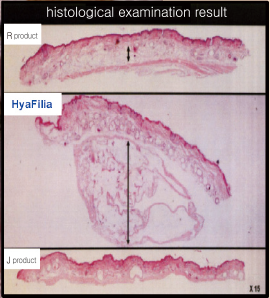
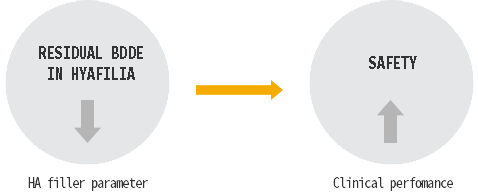
Residual BDDE is not detected in HyaFilia
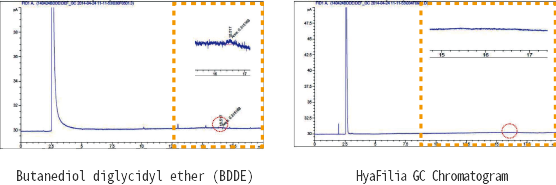
The following biocompatibility and toxicology tests were conducted on HyaFilia.
| Test Title | Test Number | Test Standards | Test Results |
|---|---|---|---|
| Acute Systemic Toxicity Test | SNUH 0800401 | ISO 10993-11 | Pass |
| Subchronic Subcutaneous Toxicity and Implantation Test in Rat - 13 Weeks |
SNUH 0800402 | ISO 10993-11 ISO 10993-6 |
Pass |
| Cytotoxicity Test | SNUH 0800403 | ISO 10993-5 | Pass |
| The Guinea Pig Maximization Test | SNUH 0800404 | ISO 10993-10 | Pass |
| Hemolysis Test | SNUH 0800405 | ISO 10993-4 | Pass |
| Intracutaneous Reactivity Test | SNUH 0800406 | ISO 10993-10 | Pass |
| Pyrogen Test | SNUH 0800407 | USP 29 | Pass |
| Bacterial Reverse Mutation Assay | SNUH 0800408 | ISO 10993-3 | Pass |
| In vitro Chromosome Aberration Test | SNUH 0800409 | ISO 10993-3 | Pass |
| In vivo Micronucleus Test | SNUH 0800410 | ISO 10993-3 | Pass |
Testing Period ~ February 2009
Testing Institution Medical Device Evaluation Center, Clinical Research Institute, Seoul National University Hospital, Korea
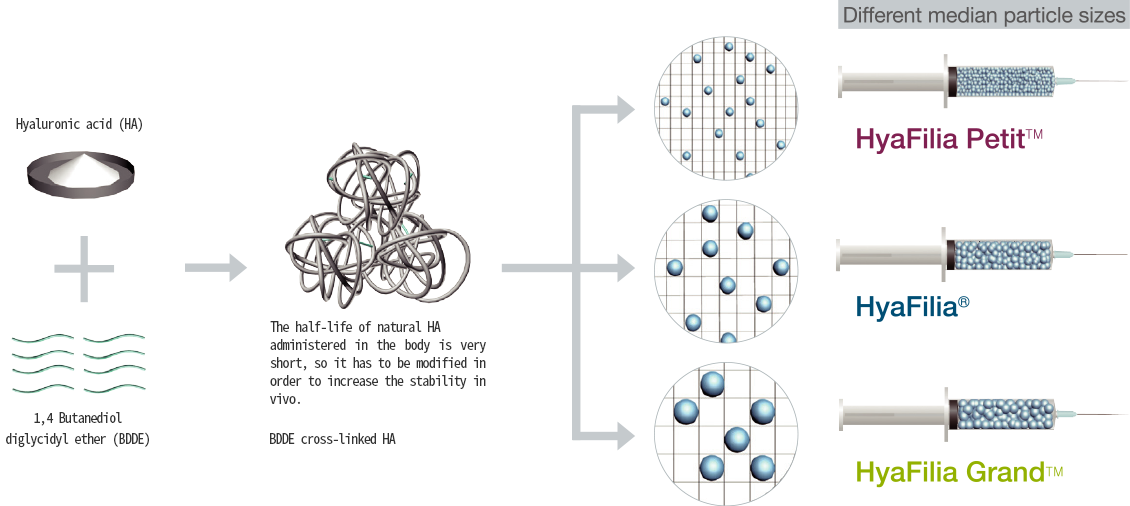
| HyaFilia Petit™ | HyaFilia® | HyaFilia Grand™ | |
|---|---|---|---|
| HyaFilia |  |
 |
 |
| Composition | Cross-linked hyaluronic acid (20mg/ml) | Cross-linked hyaluronic acid (20mg/ml) | Cross-linked hyaluronic acid (20mg/ml) |
| Average grain size | 200㎛ | 500㎛ | 1,100㎛ |
| Indications | Correction of superficial wrinkles: fine lines and wrinkles on thin skin |
Filling of all moderately pronounced wrinkles on the full face |
Filling wrinkles |
| Injection area | Superficial dermis | Mid dermis | Deep dermis or subcutaneous tissue |
| Packaging units | ·HyaFilia Petit 1.0: 1ml (27G, 29G) | ·HyaFilia Petit 1.0: 1ml (27G, 29G) | ·HyaFilia Petit 1.0: 1ml (27G, 29G) |
| Storage conditions | To be stored at room temperature (1-30℃) away from direct sunlight. | ||
| Shelf life | 36 months | ||